Project Description
Fragment-Based Discovery of Novel Non-Hydroxamate LpxC Inhibitors with Antibacterial Activity
Yamada, Y. et al., J. Med. Chem. 2020, 63, 14805
UDP-3-O-acyl-N-acetylglucosamine deacetylase (LpxC) is a zinc metalloenzyme that catalyzes the first committed step in the biosynthesis of Lipid A, an essential component of the cell envelope of Gram-negative bacteria. The most advanced, disclosed LpxC inhibitors showing antibacterial activity coordinate zinc through a hydroxamate moiety with concerns about binding to other metalloenzymes.
A collaboration between Vernalis Research and Taisho Pharmaceuticals used fragment and structure-based methods for the discovery and optimization of two series of compounds with differing modes of zinc coordination. A series was evolved from a fragment where a glycine moiety complexes zinc, which achieved low nanomolar potency in an enzyme functional assay but poor antibacterial activity on cell cultures. A second series was based on a fragment that coordinated zinc through an imidazole moiety. Structure-guided design led to a 2-(1S-hydroxyethyl)-imidazole derivative 43 exhibiting low nanomolar inhibition of LpxC and a minimum inhibitory concentration (MIC) of 4 μg/mL against Pseudomonas aeruginosa, which is little affected by the presence of albumin.
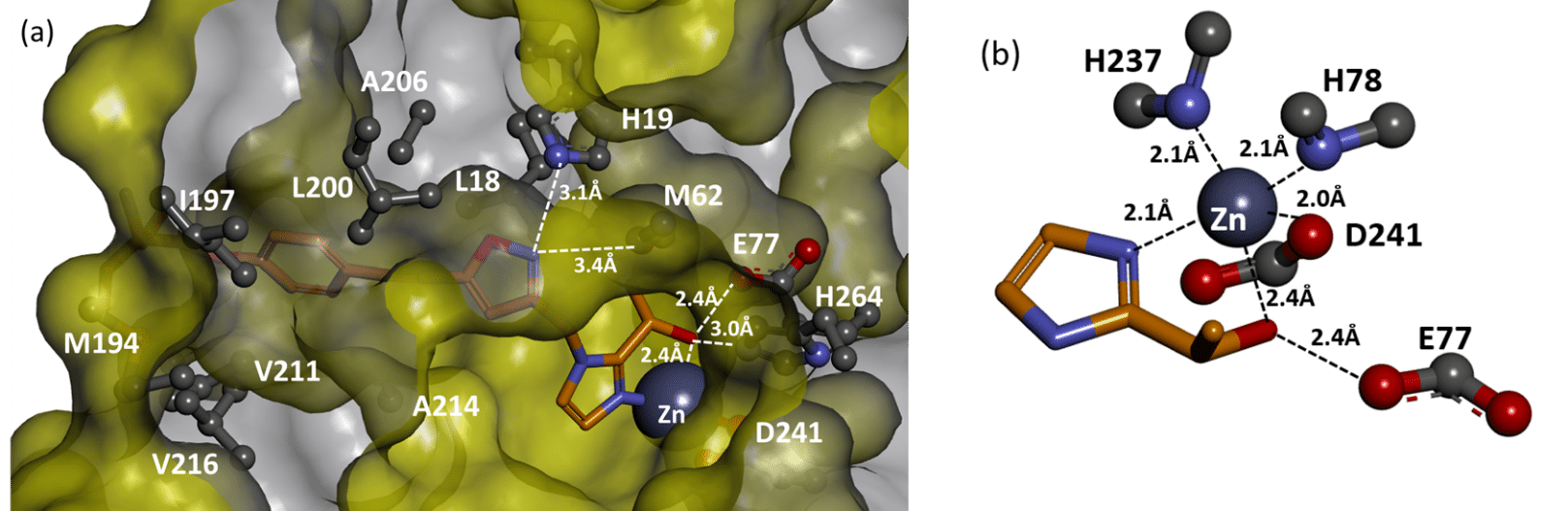
(a) Detail of the crystal structure of 43 binding to the active site of PaLpxC (b) Zinc coordination to the selected ligand and protein side chains
